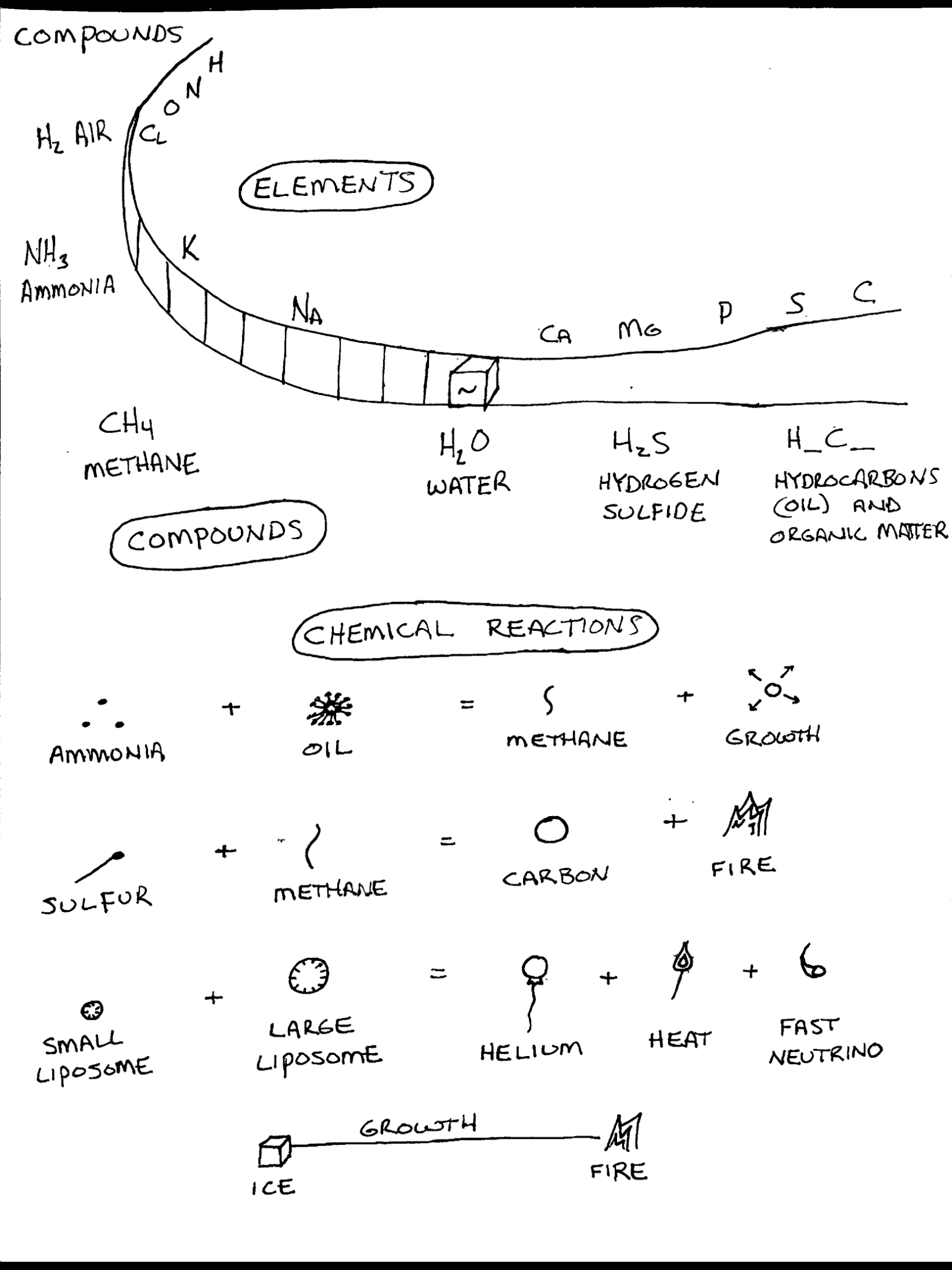
COMPOUNDS
Hydrogen is the most abundant element in the universe and bonds with many elements to form compounds. Here are some of the most abundant hydrogen compounds:
Air H2 H2 bonds with many things, it’s the glue
Water H2O is the fluid from which all life is birthed
Ammonia NH3 digestion
Hydrogen sulfide H2S flammability
Methane CH4 it is what is released into the air, as a byproduct of metabolism
Hydrocarbons, are structures which compose the material of living organisms. There are many (C_H_)
Nature managed to contain these components into a membrane and in doing so assembled a self-sustaining machine. This organism contained all the elements to breathe, eat and reproduce itself faster than it decayed. This machine is bacteria.
CHEMICAL REACTIONS
Let’s look at a few chemical reactions necessary to sustain living organisms.
GROWTH
Ammonia + oil (hydrocarbons) = Methane + Growth + hydrogen
Organisms digest food, gain energy to grow, and release methane, gas into the atmosphere.
POLARITY
Liposome + Large Liposome (deuteurium) = Helium + Fusion + Heat
When a liposome enters the proximity of a larger liposome, in this case the earth, fusion takes place. Fusion produces energy and releases helium particles and heat, regulating temperature of the organism.
FIRE
Sulfur + methane = Fire + carbon
Sulfur is a flammable solid. Methane is a flammable gas. Together they make fire, heat, and leave carbon, part of the material which composes lifeforms, which may be recycled and again filled with fresh photons.
Each of these reactions release H2 particles which bond again with other elements and continue the process. These are some of the abundant compounds and chemical reactions essential for maintaining life on earth.